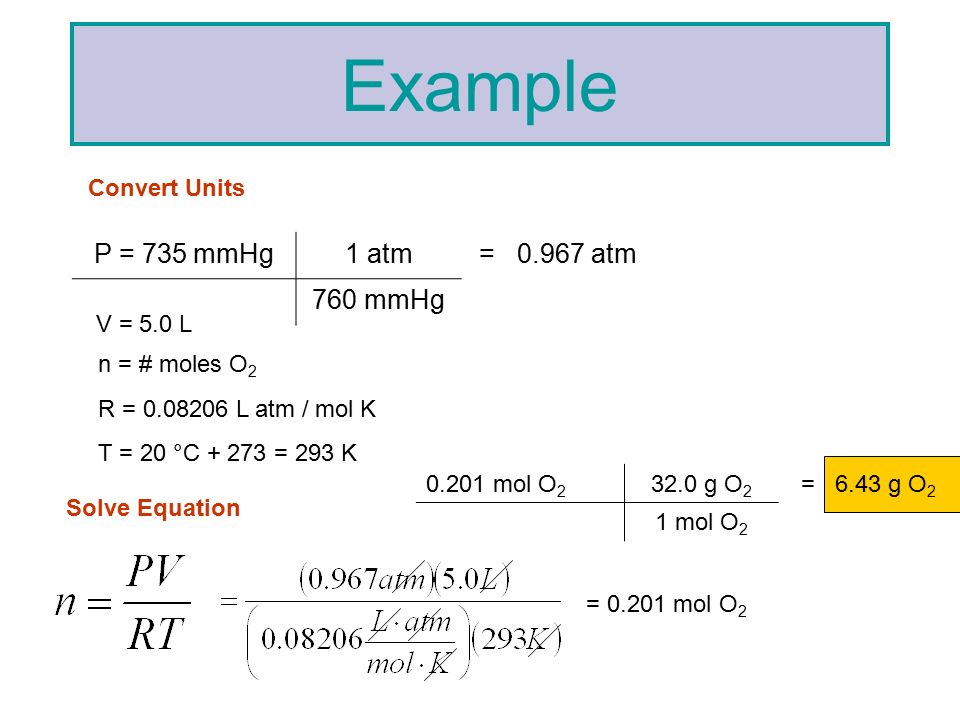
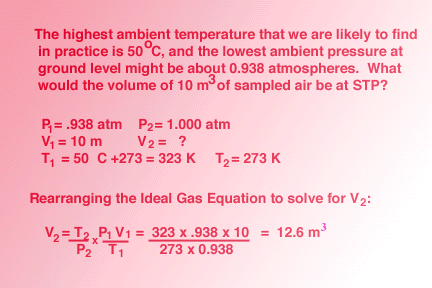
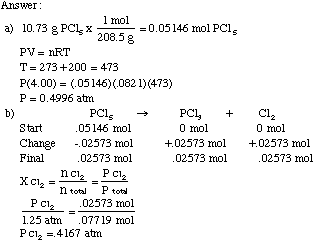
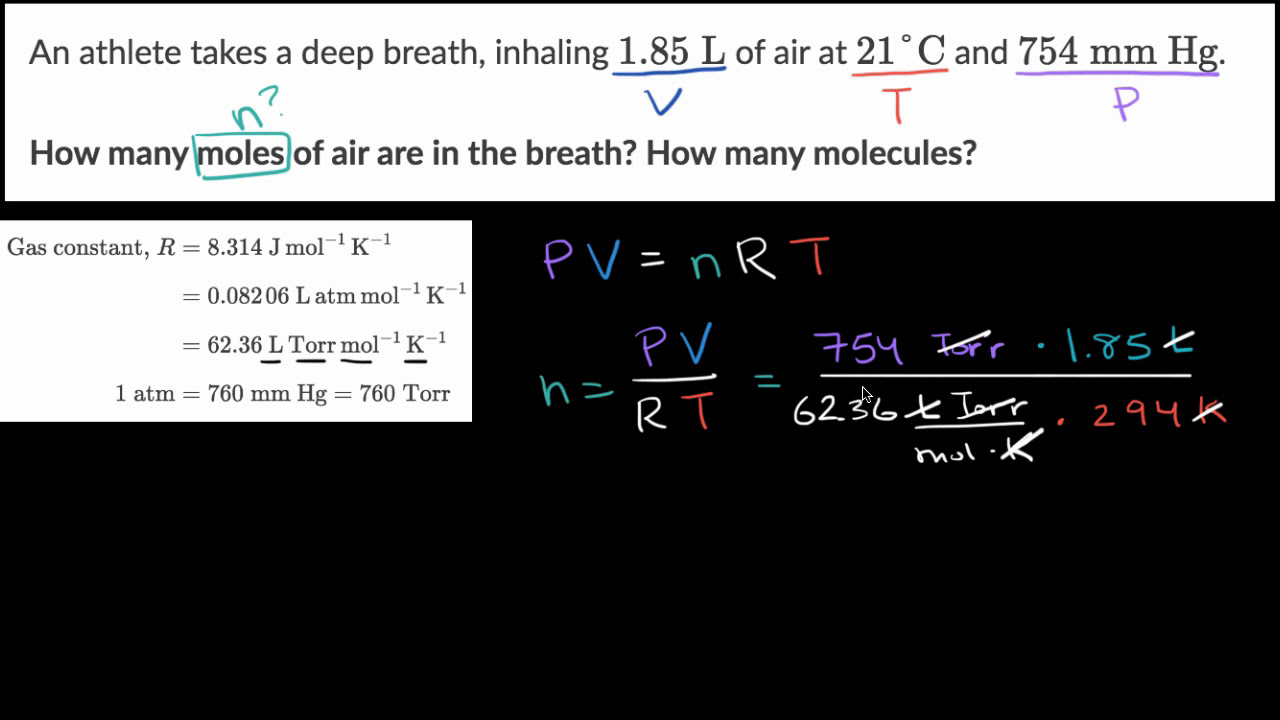
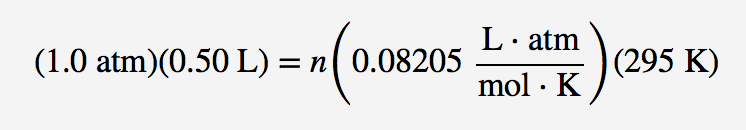Calculate the temperature of 4.0 moles of a gas occupying 5 dm^3 at 3.32 bar (R = 0.083 bar dm^3 K^-1 mol^-1) .

6.3: Combining the Gas Laws: The Ideal Gas Equation and the General Gas Equation - Chemistry LibreTexts
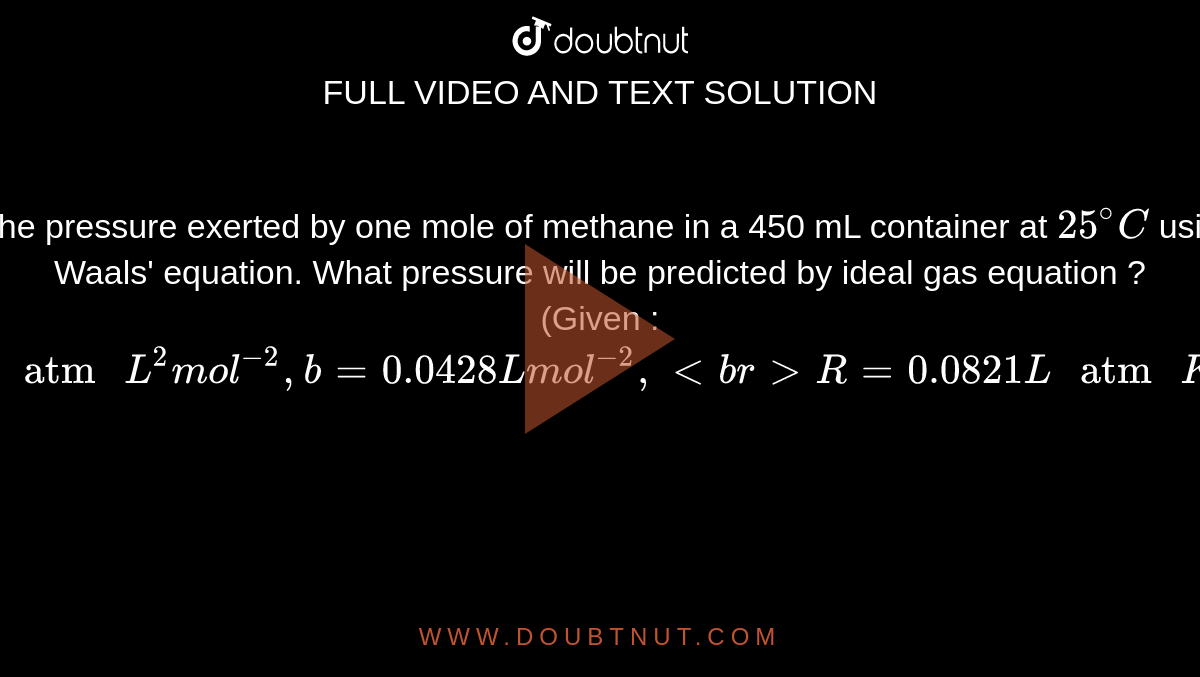
Calculate the pressure exerted by one mole of methane in a 450 mL container at 25^@C using van der Waals' equation. What pressure will be predicted by ideal gas equation ? (Given :
Five moles of ideal gas expand isothermally and reversibly from pressure 10 ATM to 2 ATM at 300 K. What is the largest mass which can be lifted through a height of

OpenStax College Physics Solution, Chapter 13, Problem 28 (Problems & Exercises) | OpenStax College Physics Answers

Ideal Gas Law Calculator (Pressure–Volume–Temperature–Amount) • Thermodynamics — Heat • Online Unit Converters