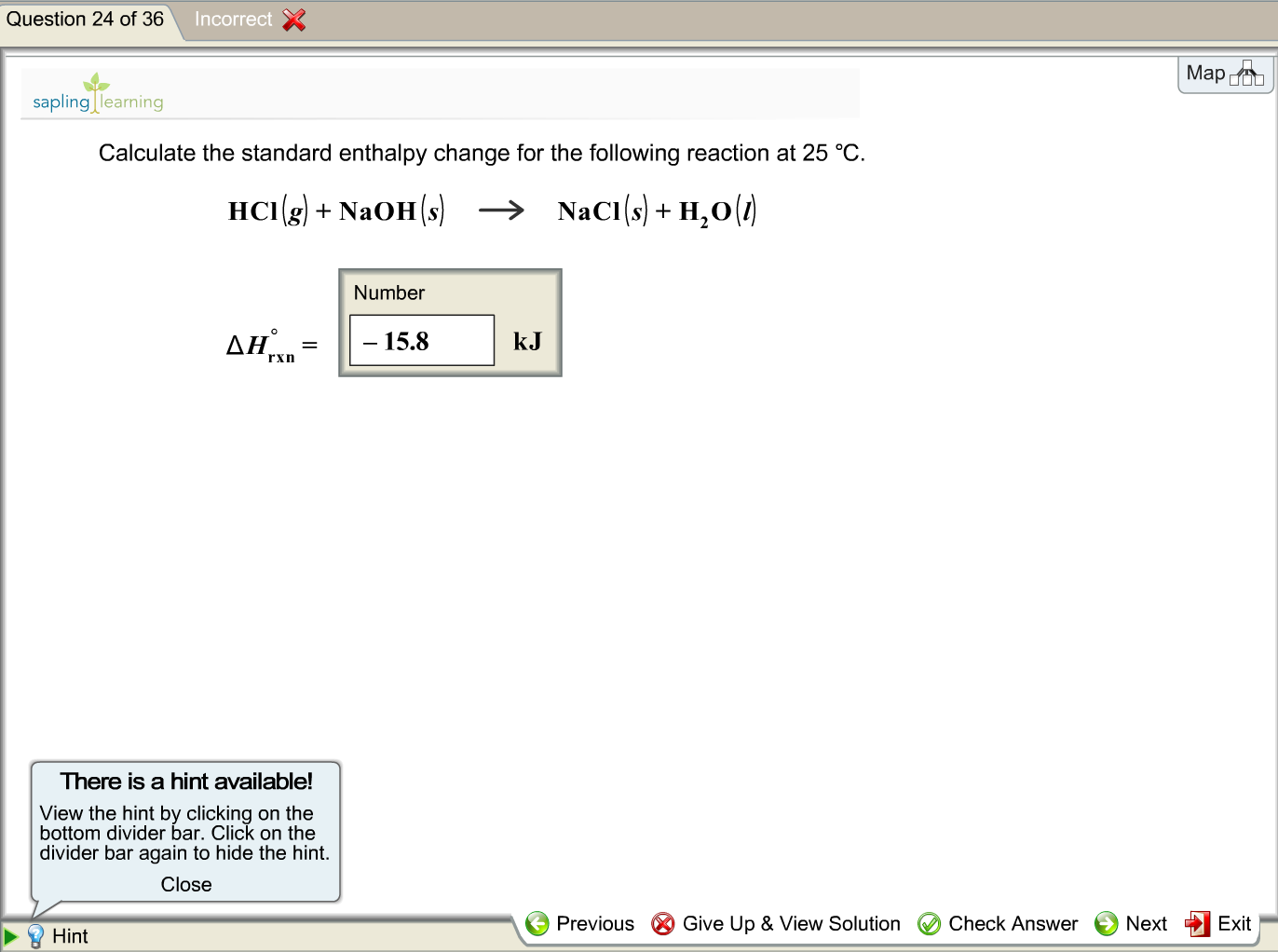
How would you calculate the standard enthalpy change for the following reaction at 25 °C: H2O (g) + C (graphite)(s) --> H2 (g) + CO (g)? | Socratic
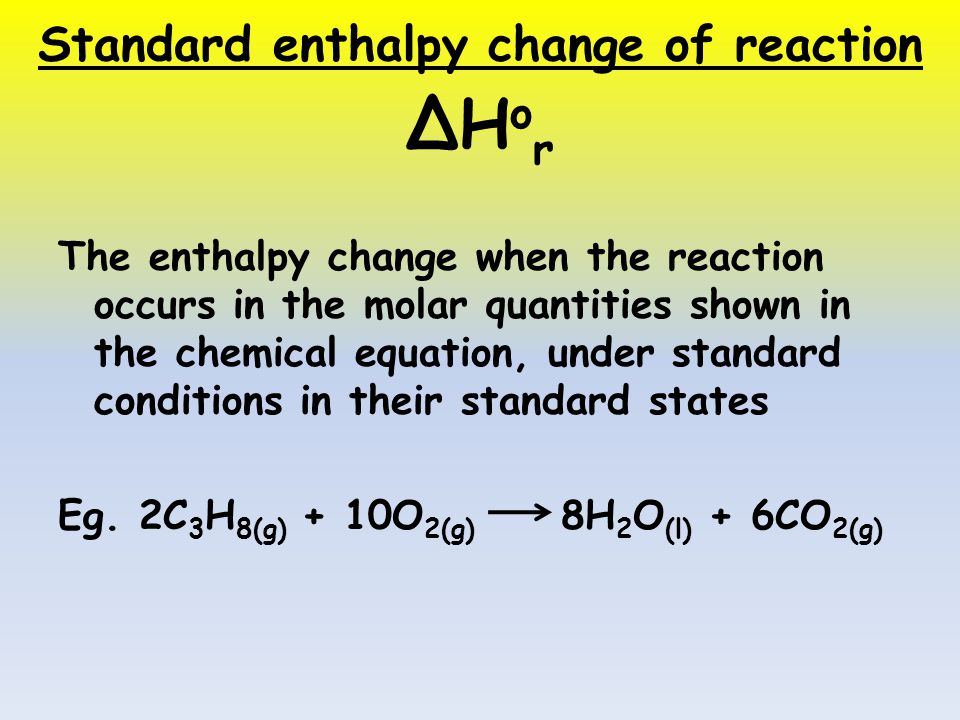
Standard Enthalpies Objectives Recall the definition of standard enthalpy changes of reaction, formation, combustion Edexcel AS Chemistry p ppt download

Question Video: Determining a Standard Enthalpy Change Given the Standard Enthlapy of Fusion | Nagwa

How would you calculate the standard enthalpy change for the following reaction at 25 °C: H2O (g) + C (graphite)(s) --> H2 (g) + CO (g)? | Socratic
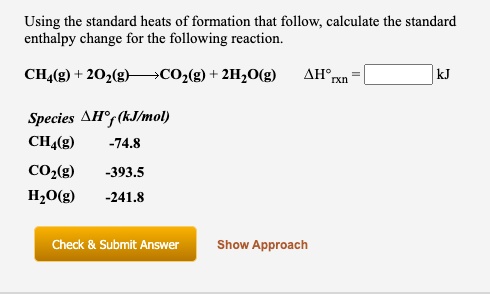
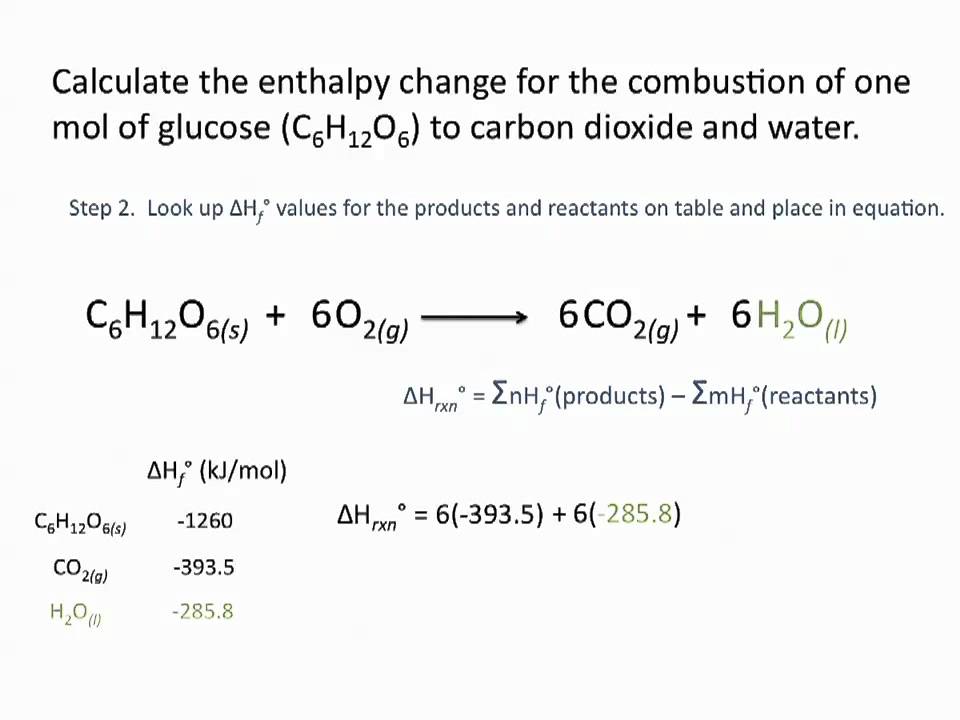
SOLVED: Using the standard heats of formation that follow; calculate the standard enthalpy change for the following reaction. CHy(g) 202(g) COz(g) 2H,O(g) AHCrxn Species AH (kJlmol) CHy(g) -74.8 COz(g) 393.5 HzO(g) 241.8

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (
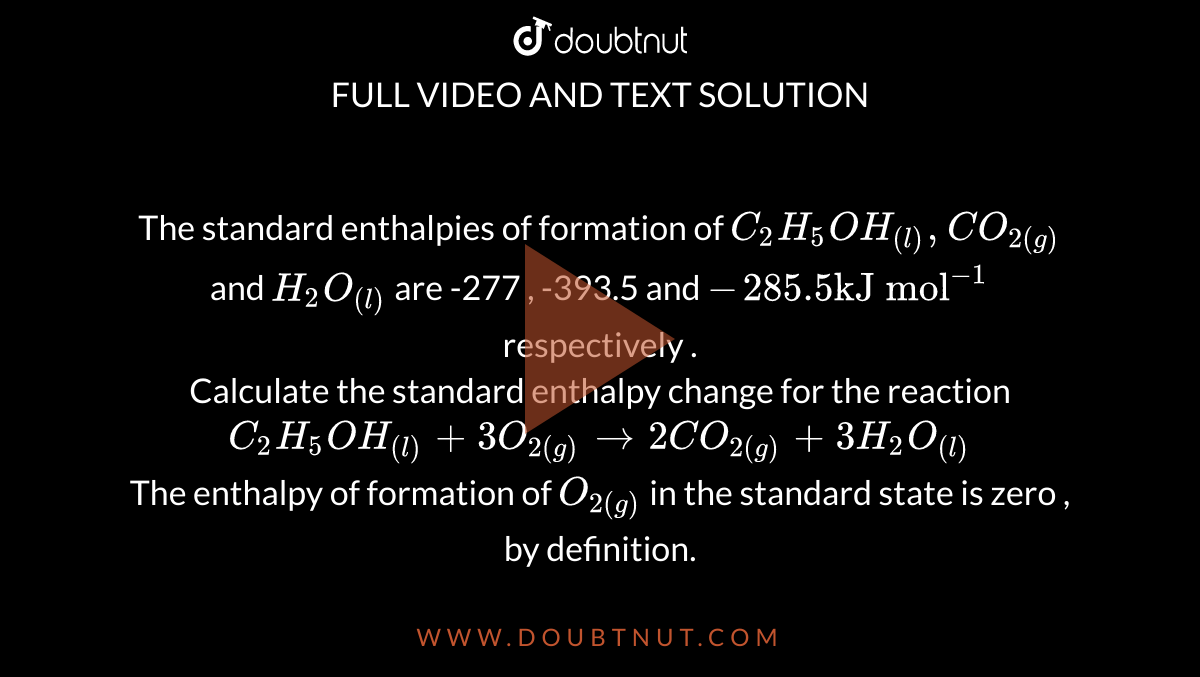
The standard enthalpies of formation of C2H5OH((l)), CO(2(g)) and H2O((l)) are -277 , -393.5 and -285.5 "kJ mol"^(-1) respectively . Calculate the standard enthalpy change for the reaction C2H5OH((l)) +3O(2(g)) to 2CO(2(g)) +

Calculate the Standard Enthalpy of the Reaction,From the Following δH° Values - Chemistry | Shaalaa.com
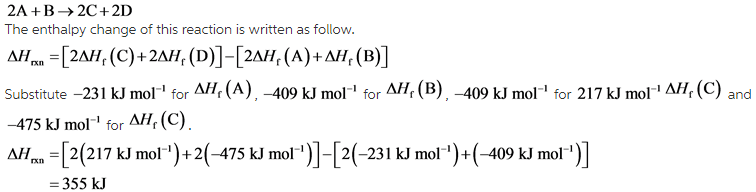
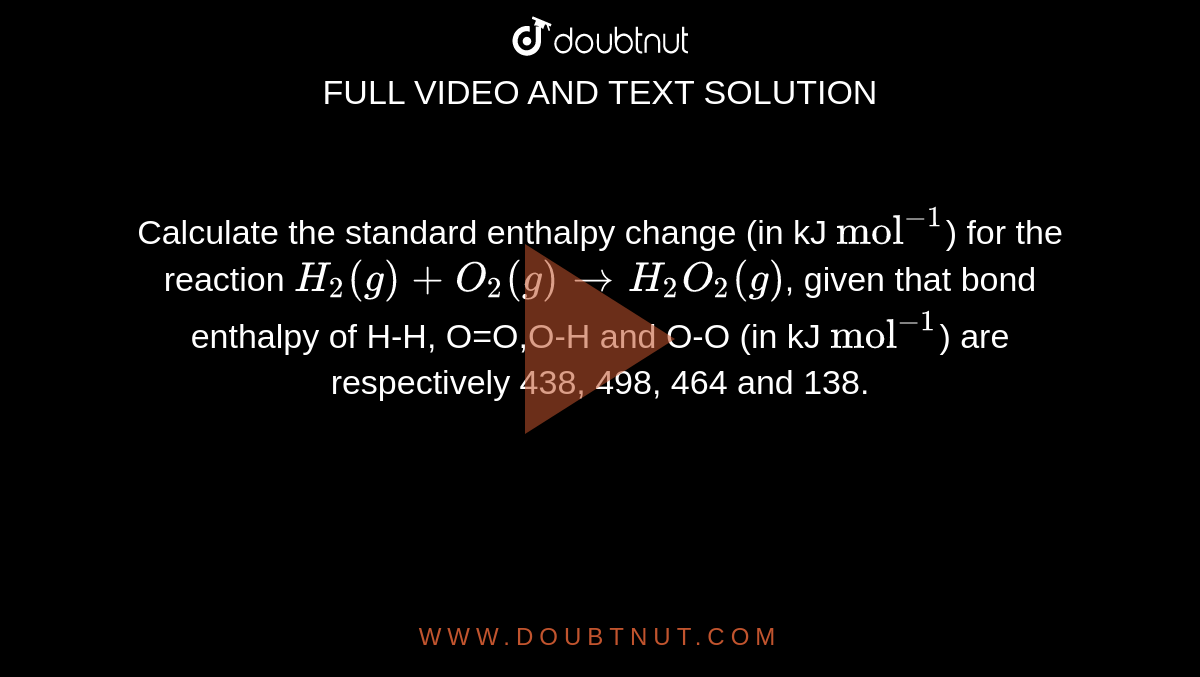
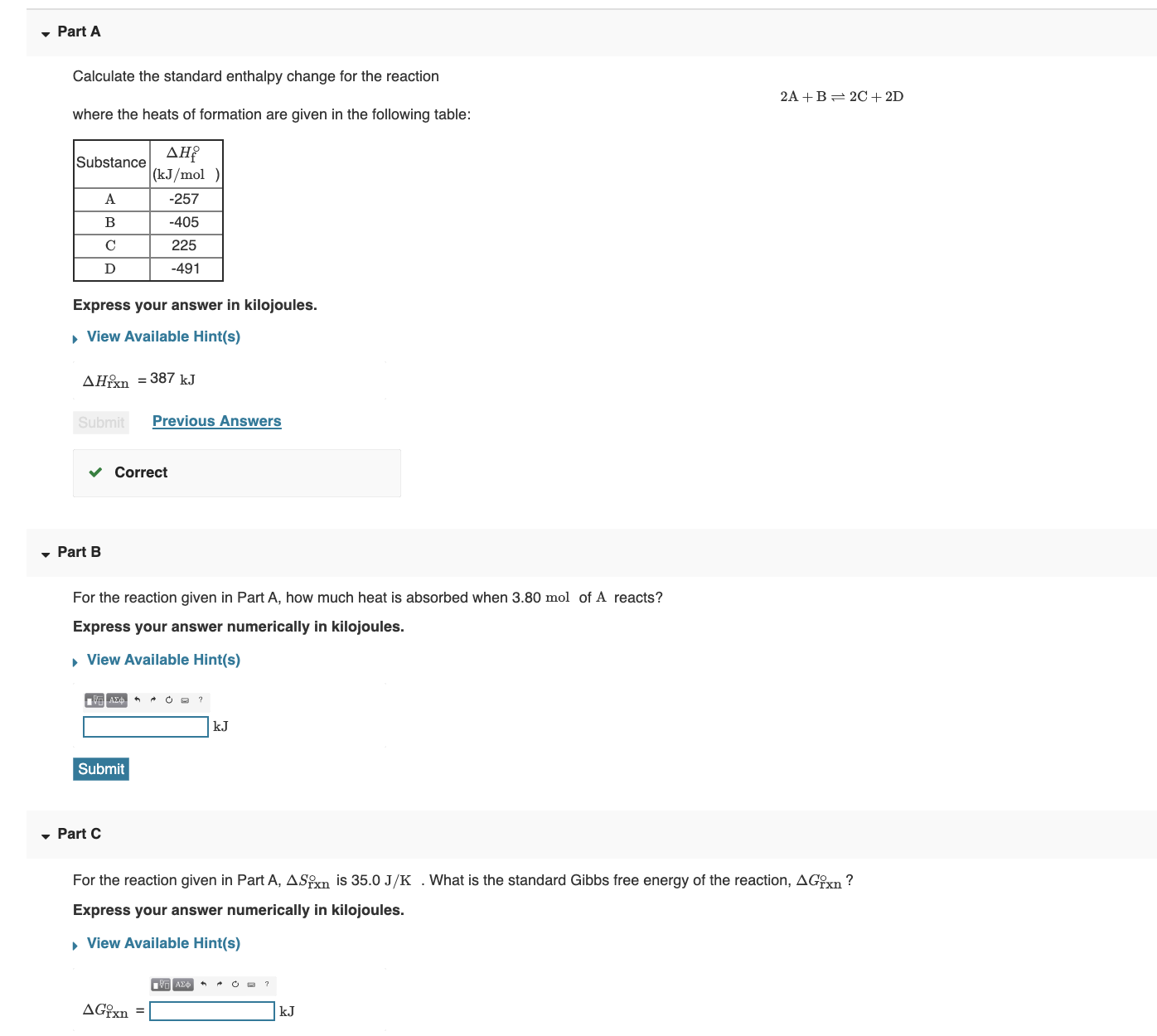
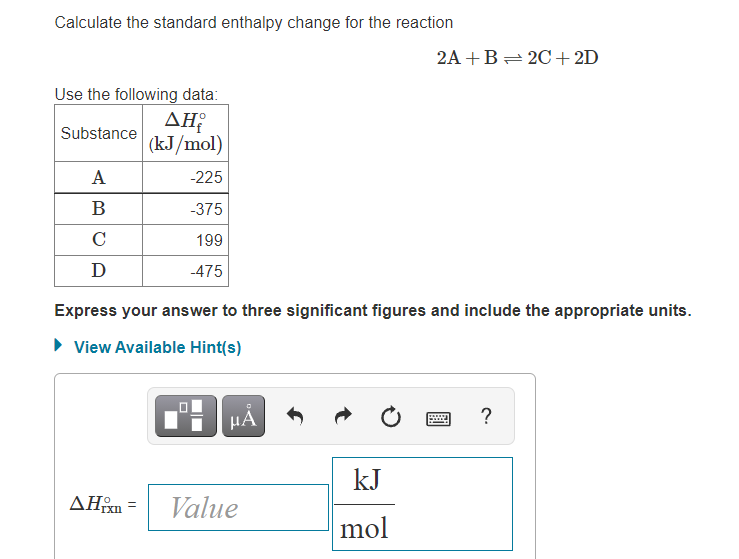
Calculate the standard enthalpy change for the reaction: 2A+B <===> 2C+2D - Home Work Help - Learn CBSE Forum

If the standard internal energy change for the reaction OF2(g) + H2O(g)⟶ O2(g) + 2HF(g) , at 298 K is x kJ . [Given standard enthalpies of formation in kJ mol^-1 are